VAMP2
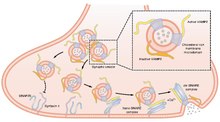
Vesicle-associated membrane protein 2 (VAMP2) is a protein that in humans is encoded by the VAMP2 gene.[5][6]
Function
[edit]Synaptobrevins/VAMPs, syntaxins, and the 25-kD synaptosomal-associated protein SNAP25 are the main components of a protein complex involved in the docking and/or fusion of synaptic vesicles with the presynaptic membrane. VAMP2 is a member of the vesicle-associated membrane protein (VAMP)/synaptobrevin family. VAMP2 is thought to participate in neurotransmitter release at a step between docking and fusion. Mice lacking functional synaptobrevin2/VAMP2 gene cannot survive after birth, and have a dramatically reduced synaptic transmission, around 10% of control.[7] The protein forms a stable complex with syntaxin, synaptosomal-associated protein, 25 kD, and complexin. It also forms a distinct complex with synaptophysin.[6]
Clinical significance
[edit]Heterozygous mutations in VAMP2 cause a neurodevelopmental disorder with hypotonia and autistic features (with or without hyperkinetic movements).[8][9][10]
Interactions
[edit]VAMP2 has been shown to interact with:
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000220205 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000020894 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Archer BT, Ozçelik T, Jahn R, Francke U, Südhof TC (Oct 1990). "Structures and chromosomal localizations of two human genes encoding synaptobrevins 1 and 2". The Journal of Biological Chemistry. 265 (28): 17267–73. doi:10.1016/S0021-9258(17)44898-8. PMID 1976629.
- ^ a b "Entrez Gene: VAMP2 vesicle-associated membrane protein 2 (synaptobrevin 2)".
- ^ Schoch S, Deák F, Königstorfer A, Mozhayeva M, Sara Y, Südhof TC, Kavalali ET (Nov 2001). "SNARE function analyzed in synaptobrevin/VAMP knockout mice". Science. 294 (5544): 1117–22. Bibcode:2001Sci...294.1117S. doi:10.1126/science.1064335. PMID 11691998. S2CID 40321111.
- ^ Salpietro V, Malintan NT, Llano-Rivas I, et al. (Apr 2019). "Mutations in the Neuronal Vesicular SNARE VAMP2 Affect Synaptic Membrane Fusion and Impair Human Neurodevelopment". The American Journal of Human Genetics. 104 (4): 721–730. doi:10.1016/j.ajhg.2019.02.016. PMC 6451933. PMID 30929742.
- ^ Sunaga Y, Muramatsu K, Kosaki K, et al. (Apr 2020). "Variant in the neuronal vesicular SNARE VAMP2 (synaptobrevin-2): First report in Japan". Brain and Development. 42 (7): 529–533. doi:10.1016/j.braindev.2020.04.001. PMID 32336483. S2CID 216095891.
- ^ "OMIM entry: Neurodevelopmental disorder with hypotonia and autistic features with or without hyperkinetic movements".
- ^ Martincic I, Peralta ME, Ngsee JK (Oct 1997). "Isolation and characterization of a dual prenylated Rab and VAMP2 receptor". The Journal of Biological Chemistry. 272 (43): 26991–8. doi:10.1074/jbc.272.43.26991. PMID 9341137.
- ^ Li Y, Chin LS, Weigel C, Li L (Nov 2001). "Spring, a novel RING finger protein that regulates synaptic vesicle exocytosis". The Journal of Biological Chemistry. 276 (44): 40824–33. doi:10.1074/jbc.M106141200. PMID 11524423.
- ^ a b Hao JC, Salem N, Peng XR, Kelly RB, Bennett MK (Mar 1997). "Effect of mutations in vesicle-associated membrane protein (VAMP) on the assembly of multimeric protein complexes". The Journal of Neuroscience. 17 (5): 1596–603. doi:10.1523/JNEUROSCI.17-05-01596.1997. PMC 6573372. PMID 9030619.
- ^ a b Chen X, Tomchick DR, Kovrigin E, Araç D, Machius M, Südhof TC, Rizo J (Jan 2002). "Three-dimensional structure of the complexin/SNARE complex". Neuron. 33 (3): 397–409. doi:10.1016/s0896-6273(02)00583-4. PMID 11832227. S2CID 17878965.
- ^ a b Paumet F, Le Mao J, Martin S, Galli T, David B, Blank U, Roa M (Jun 2000). "Soluble NSF attachment protein receptors (SNAREs) in RBL-2H3 mast cells: functional role of syntaxin 4 in exocytosis and identification of a vesicle-associated membrane protein 8-containing secretory compartment". Journal of Immunology. 164 (11): 5850–7. doi:10.4049/jimmunol.164.11.5850. PMID 10820264.
- ^ Imai A, Nashida T, Yoshie S, Shimomura H (Aug 2003). "Intracellular localisation of SNARE proteins in rat parotid acinar cells: SNARE complexes on the apical plasma membrane". Archives of Oral Biology. 48 (8): 597–604. doi:10.1016/s0003-9969(03)00116-x. PMID 12828989.
- ^ Kawanishi M, Tamori Y, Okazawa H, Araki S, Shinoda H, Kasuga M (Mar 2000). "Role of SNAP23 in insulin-induced translocation of GLUT4 in 3T3-L1 adipocytes. Mediation of complex formation between syntaxin4 and VAMP2". The Journal of Biological Chemistry. 275 (11): 8240–7. doi:10.1074/jbc.275.11.8240. PMID 10713150.
- ^ Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Südhof TC, Rizo J (Aug 1999). "A conformational switch in syntaxin during exocytosis: role of munc18". The EMBO Journal. 18 (16): 4372–82. doi:10.1093/emboj/18.16.4372. PMC 1171512. PMID 10449403.
- ^ McMahon HT, Missler M, Li C, Südhof TC (Oct 1995). "Complexins: cytosolic proteins that regulate SNAP receptor function". Cell. 83 (1): 111–9. doi:10.1016/0092-8674(95)90239-2. PMID 7553862. S2CID 675343.
- ^ Pérez-Brangulí F, Muhaisen A, Blasi J (Jun 2002). "Munc 18a binding to syntaxin 1A and 1B isoforms defines its localization at the plasma membrane and blocks SNARE assembly in a three-hybrid system assay". Molecular and Cellular Neurosciences. 20 (2): 169–80. doi:10.1006/mcne.2002.1122. PMID 12093152. S2CID 23927545.
- ^ Margittai M, Otto H, Jahn R (Mar 1999). "A stable interaction between syntaxin 1a and synaptobrevin 2 mediated by their transmembrane domains". FEBS Letters. 446 (1): 40–4. doi:10.1016/s0014-5793(99)00028-9. PMID 10100611. S2CID 9115709.
- ^ Mollinedo F, Martín-Martín B, Calafat J, Nabokina SM, Lazo PA (Jan 2003). "Role of vesicle-associated membrane protein-2, through Q-soluble N-ethylmaleimide-sensitive factor attachment protein receptor/R-soluble N-ethylmaleimide-sensitive factor attachment protein receptor interaction, in the exocytosis of specific and tertiary granules of human neutrophils". Journal of Immunology. 170 (2): 1034–42. doi:10.4049/jimmunol.170.2.1034. PMID 12517971.
- ^ Jagadish MN, Fernandez CS, Hewish DR, Macaulay SL, Gough KH, Grusovin J, Verkuylen A, Cosgrove L, Alafaci A, Frenkel MJ, Ward CW (Aug 1996). "Insulin-responsive tissues contain the core complex protein SNAP-25 (synaptosomal-associated protein 25) A and B isoforms in addition to syntaxin 4 and synaptobrevins 1 and 2". The Biochemical Journal. 317. 317 (3): 945–54. doi:10.1042/bj3170945. PMC 1217577. PMID 8760387.
- ^ Reed GL, Houng AK, Fitzgerald ML (Apr 1999). "Human platelets contain SNARE proteins and a Sec1p homologue that interacts with syntaxin 4 and is phosphorylated after thrombin activation: implications for platelet secretion". Blood. 93 (8): 2617–26. doi:10.1182/blood.V93.8.2617. PMID 10194441.
Further reading
[edit]- Brumell JH, Volchuk A, Sengelov H, Borregaard N, Cieutat AM, Bainton DF, Grinstein S, Klip A (Dec 1995). "Subcellular distribution of docking/fusion proteins in neutrophils, secretory cells with multiple exocytic compartments". Journal of Immunology. 155 (12): 5750–9. doi:10.4049/jimmunol.155.12.5750. PMID 7499863. S2CID 32809167.
- Kutay U, Ahnert-Hilger G, Hartmann E, Wiedenmann B, Rapoport TA (Jan 1995). "Transport route for synaptobrevin via a novel pathway of insertion into the endoplasmic reticulum membrane". The EMBO Journal. 14 (2): 217–23. doi:10.1002/j.1460-2075.1995.tb06994.x. PMC 398073. PMID 7835332.
- Chapman ER, An S, Barton N, Jahn R (Nov 1994). "SNAP-25, a t-SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils". The Journal of Biological Chemistry. 269 (44): 27427–32. doi:10.1016/S0021-9258(18)47003-2. PMID 7961655.
- Hunt JM, Bommert K, Charlton MP, Kistner A, Habermann E, Augustine GJ, Betz H (Jun 1994). "A post-docking role for synaptobrevin in synaptic vesicle fusion". Neuron. 12 (6): 1269–79. doi:10.1016/0896-6273(94)90443-X. PMID 8011337. S2CID 799315.
- Maruyama K, Sugano S (Jan 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Jagadish MN, Fernandez CS, Hewish DR, Macaulay SL, Gough KH, Grusovin J, Verkuylen A, Cosgrove L, Alafaci A, Frenkel MJ, Ward CW (Aug 1996). "Insulin-responsive tissues contain the core complex protein SNAP-25 (synaptosomal-associated protein 25) A and B isoforms in addition to syntaxin 4 and synaptobrevins 1 and 2". The Biochemical Journal. 317. 317 (3): 945–54. doi:10.1042/bj3170945. PMC 1217577. PMID 8760387.
- Mandon B, Chou CL, Nielsen S, Knepper MA (Aug 1996). "Syntaxin-4 is localized to the apical plasma membrane of rat renal collecting duct cells: possible role in aquaporin-2 trafficking". The Journal of Clinical Investigation. 98 (4): 906–13. doi:10.1172/JCI118873. PMC 507504. PMID 8770861.
- Timmers KI, Clark AE, Omatsu-Kanbe M, Whiteheart SW, Bennett MK, Holman GD, Cushman SW (Dec 1996). "Identification of SNAP receptors in rat adipose cell membrane fractions and in SNARE complexes co-immunoprecipitated with epitope-tagged N-ethylmaleimide-sensitive fusion protein". The Biochemical Journal. 320. 320 (2): 429–36. doi:10.1042/bj3200429. PMC 1217948. PMID 8973549.
- Betz A, Okamoto M, Benseler F, Brose N (Jan 1997). "Direct interaction of the rat unc-13 homologue Munc13-1 with the N terminus of syntaxin". The Journal of Biological Chemistry. 272 (4): 2520–6. doi:10.1074/jbc.272.4.2520. PMID 8999968.
- Hao JC, Salem N, Peng XR, Kelly RB, Bennett MK (Mar 1997). "Effect of mutations in vesicle-associated membrane protein (VAMP) on the assembly of multimeric protein complexes". The Journal of Neuroscience. 17 (5): 1596–603. doi:10.1523/JNEUROSCI.17-05-01596.1997. PMC 6573372. PMID 9030619.
- Martincic I, Peralta ME, Ngsee JK (Oct 1997). "Isolation and characterization of a dual prenylated Rab and VAMP2 receptor". The Journal of Biological Chemistry. 272 (43): 26991–8. doi:10.1074/jbc.272.43.26991. PMID 9341137.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (Oct 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Weir ML, Klip A, Trimble WS (Jul 1998). "Identification of a human homologue of the vesicle-associated membrane protein (VAMP)-associated protein of 33 kDa (VAP-33): a broadly expressed protein that binds to VAMP". The Biochemical Journal. 333. 333 (2): 247–51. doi:10.1042/bj3330247. PMC 1219579. PMID 9657962.
- Isenmann S, Khew-Goodall Y, Gamble J, Vadas M, Wattenberg BW (Jul 1998). "A splice-isoform of vesicle-associated membrane protein-1 (VAMP-1) contains a mitochondrial targeting signal". Molecular Biology of the Cell. 9 (7): 1649–60. doi:10.1091/mbc.9.7.1649. PMC 25402. PMID 9658161.
- Prekeris R, Klumperman J, Chen YA, Scheller RH (Nov 1998). "Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes". The Journal of Cell Biology. 143 (4): 957–71. doi:10.1083/jcb.143.4.957. PMC 2132958. PMID 9817754.
- Nishimura Y, Hayashi M, Inada H, Tanaka T (Jan 1999). "Molecular cloning and characterization of mammalian homologues of vesicle-associated membrane protein-associated (VAMP-associated) proteins". Biochemical and Biophysical Research Communications. 254 (1): 21–6. doi:10.1006/bbrc.1998.9876. PMID 9920726.
- Valdez AC, Cabaniols JP, Brown MJ, Roche PA (Mar 1999). "Syntaxin 11 is associated with SNAP-23 on late endosomes and the trans-Golgi network". Journal of Cell Science. 112. 112 (6): 845–54. doi:10.1242/jcs.112.6.845. PMID 10036234.
- Margittai M, Otto H, Jahn R (Mar 1999). "A stable interaction between syntaxin 1a and synaptobrevin 2 mediated by their transmembrane domains". FEBS Letters. 446 (1): 40–4. doi:10.1016/S0014-5793(99)00028-9. PMID 10100611. S2CID 9115709.
- Fasshauer D, Antonin W, Margittai M, Pabst S, Jahn R (May 1999). "Mixed and non-cognate SNARE complexes. Characterization of assembly and biophysical properties". The Journal of Biological Chemistry. 274 (22): 15440–6. doi:10.1074/jbc.274.22.15440. PMID 10336434.











